SG Diagnostics COVID-19 Antigen Rapid Test Kit (Self-Test)

Overview of the Virus
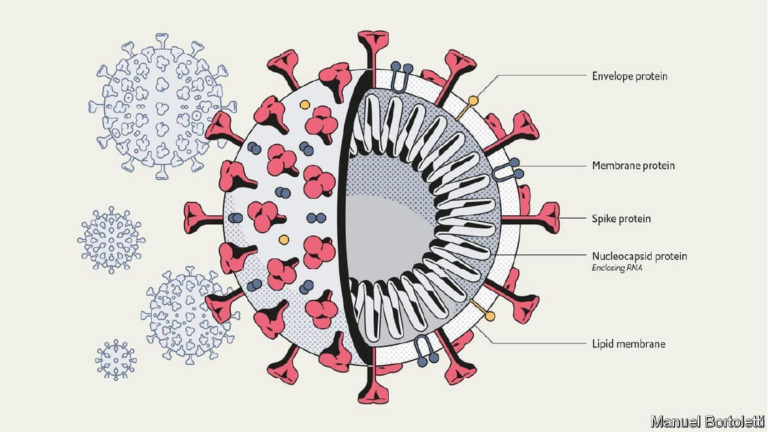
The novel coronavirus SARS-COV-2 is the causative pathogen for the global pandemic of COVID-19 that has spread to 219 countries. Most infected people will experience mild to severe respiratory illness and recover without special treatment. The most common symptoms are fever, cough and fatigue. Older people and those with underlying medical problems (e.g. cardiovascular disease, diabetes, chronic respiratory disease and cancer) are more likely to develop serious illness and serious symptoms include difficulty breathing or shortness of breath, chest pain and loss of speech or movement. It typically takes 5 – 6 days for someone that is infected with the virus for symptoms to appear but it can take up to 14 days in some individuals.
The SG Diagnostics COVID-19 Antigen Rapid Test Kit is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 spike and nucleocapsid protein antigens from swab samples via the double antibody sandwich method. After loading, the sample will diffuse upwards from the charging end by capillary action, and then the SARS-CoV-2 spike and nucleocapsid protein antigens in the sample will combine with the antibody in the marker pad to form colloidal gold antibody-antigen complex; the complex continues to diffuse upwards to the nitrocellulose membrane with the sample, and then blocked by Test (T) Line packed with antibody to form colloidal gold labeled antibody-antigen-immune complex. The rest unblocked colloidal gold complex continues to move upwards and combine with the Control (C) line (quality control line), indicating that the reaction is completed.
Features and Benefits

Reliable Results
- The assay time is shorter (within 10 minutes as compared to around 24 hours for enzyme-linked immunosorbent assay (ELISA))
- The cost is cheaper than ELISA
- The test can be carried out at the point-of-care
- It is a one-step assay that requires no special skills or instrumentation to achieve the result(s)
- It has a long shelf life and assay reagents do not require refrigeration or freezer storage
- The samples do not need to be pre-treated before applying to the assay
Testing Instructions
Step 1: Collect Swab Sample
Option 1) nasal swab sample
Insert the swab about 2.5cm into the nostril and rotate the swab 5 times. Repeat the process in the other nostril and withdraw.
Option 2) oropharyngeal (throat) swab sample
Open your mouth wide and stick out your tongue. Try to swipe the soft tip of the swab across the very back of your tongue, close to your tonsils, at least 5 times.
Step 2: Stir Swab
Note: For best performance, sample should be eluted from the swab within 1 hour after collection and be tested as soon as possible afterwards.- Remove the white cap from the elution tube and insert the swab into it.
- Roll the swab at least 10 times while pressing the swab tip against the bottom and side of the elution tube.
- Squeeze or press the swab against the side of the tube to remove as much liquid as possible, then dispose the swab.
- Cover the elution tube tightly with the nozzle connected to the tube.

Step 3: Run Test
Note: The test cassette must be at room temperature before use, and the test must be operated at room temperature.
- Open the test cassette package and lay it flat.
- Add 4 drops of the test sample into the sample well and wait for the coloured band to appear.
- Start and timer and read the results after 10 minutes. The result is invalid after 15 minutes.
- Place the used Swab, Sample Eluent Vial and Test Cassette in a plastic disposal bag and dispose the filled bag in your trash bin.

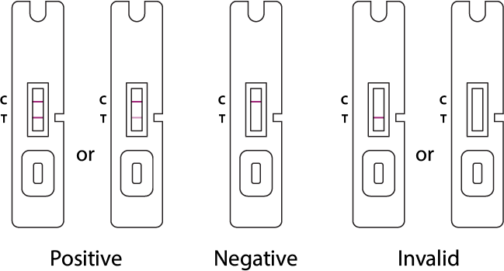
Result Interpretation

The test results are analyzed as follows:
Negative
A negative specimen will give a single red colored Control Line in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected.
Positive
A positive specimen will give two red colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint sample Line. Any visible red colored Sample Line is positive.
Invalid
If no lines are seen, if just the red Test Line is seen, or if the red Control Line is not seen, the assay is invalid. Invalid tests should be repeated.